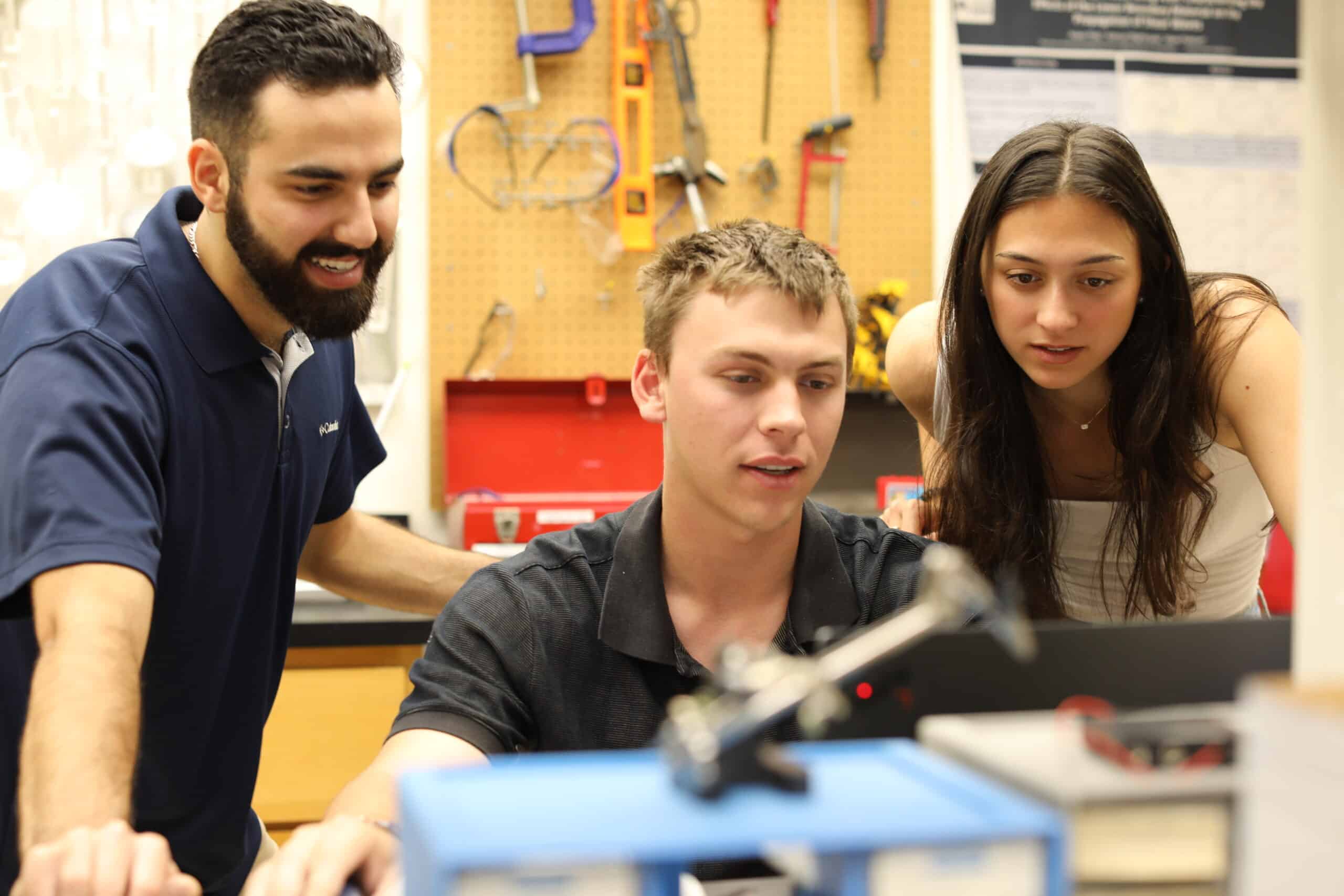
BME Design Fellows
As a fellow, you’ll design and test a medical instrument based on a clinical need identified by a Duke Health clinician.
Academics
The courses in program expose students to:
- Low- medium- and high-fidelity prototyping
- Electronic and mechanical computer-aided design
- Printed circuit board layout and fabrication
- Electronic signal processing in hardware and with microcontrollers
- The FDA medical device clearance process
-
Also known as First-Year Design, EGR101L provides students with the knowledge and experience needed to become successful engineers. The course is required for all Duke Engineering undergraduates.
EGR101L inverts the traditional teaching method by delivering lectures outside of the class (topic-based videos, student team videos, online quizzes) and moving “homework” into the class with in-class activities which are hands-on and collaborative.
This flipped-classroom requires students to come prepared for class time in order to be able to participate in relevant topic exercises in groups.
-
Recommended for sophomore or junior year; counts as general BME elective. Skills learned that are included in the syllabus include:
- CAD using OnShape
- 3D printing
- ECAD (schematic capture, PCB layout & fabrication) using KiCad
- Introductory analog & digital circuit design
- PCB component population (soldering)
- Git version control
- Microcontroller firmware development using the Visual Studio Code IDE:
- State Machines
- CI testing
- Electronics specification testing with oscilloscopes and multimeters
- Agile project management, Kanban boards and Gantt charts
-
Choose from:
BME 473L/474L Medical Device Design I & II (Dr. Kyle – 473L can count as a BME general elective or IM advanced elective, and 474L counts as the design requirement)
or
One-semester capstone design:
- Biophotonic Instrumentation (BME463L) (Dr. Dhalla)
- Medical Instrument Design (BME464L) (Dr. von Ramm)
-
-
BME547 Medical Software Design (Dr. Ward or Palmeri)
Can count as BME general elective or IM advanced elective - BME554L Embedded Medical Devices (Dr. Palmeri)
- BME590 Quality Systems for Biomedical Engineering (K. Fearis)
- BME590L Advanced Design & Manufacturing (P. Fearis)
- BME500 Regulation and Reimbursement of Medical Products: Practice & Policy (Prof. Silcox)
- BME590: Intro to Surgical Medical Robotics (xlist ME 555)
- BME590: Systems Engineering in Global Health (Dr. Richardson)
- BME432L: Biomechanics Vehicle Safety (Dr. Luck)
- BME462L: Design for the Developing World (Dr. Madonna or Dr. Ramanujam)
-
Design Project Independent Study
Requires pre-approval by Dr. Mark Palmeri - One semester of Design Health (Dr. Richardson)
NOTE: One advanced design elective is required if you take BME473L/474L; otherwise, if you choose to take a 1-semester capstone design course, then two advanced electives are required.
-
Preventing Hearing Loss in Children
When Duke global health researchers identified a need for a hearing-loss screening tool, the BME Design Fellows program swung into action. The work resulted in a prototype to make tympanometry accessible, affordable, and easy to use.
Faculty Advisors

Mark L. Palmeri
Professor of the Practice in the Department of Biomedical Engineering

Aaron M Kyle
Professor of the Practice in the Department of Biomedical Engineering
Opportunity in Duke University Medical Center and Local Industry
We have an active LinkedIn group where internship and job opportunities are commonly shared and current/past design fellow experiences are highlighted.
Students who participate in this program frequently engage in summer internships with biomedical engineering companies, both inside and outside Research Triangle Park (RTP), including:
- Blur
- Garmin
- Stryker
- InnAVasc (acquired by W.L. Gore & Associates)
- Edwards Lifesciences
- MicroElastic Ultrasound
- Zimmer Biomet
- Pilleve
- LivaNova
- Boundless Science
BME Design Fellows have had opportunities to design medical devices in various units and departments within Duke’s esteemed medical center, including:
- Duke University Hospital
- Surgery
- Gastroenterology
- Urology
- Neurology
- Pediatrics
- School of Nursing
